Gas Facts
The Earth’s atmosphere and the air you breathe are made up of gases. How much do you know about this interesting state of matter?
If you answered, “Not much,” keep reading to learn more!

What Are Gases?
Gases are one of the three main states of matter. The other two are liquids and solids.
First, let’s back up a little. What is matter? Matter is any physical substance that takes up space and has mass.
Matter is made up of millions of tiny atoms and molecules that combine to form objects like desks, cars, planets, and even you! Matter exists mostly in the three states listed above, and today we are focusing on gas.
Gas molecules are extra spread out and very energetic, constantly moving around. Gases are mostly invisible and have no fixed shape. The air we breathe is made up of gases like nitrogen and oxygen.
If you put a gas inside of a container, it will spread out to fill the entire volume of the container, no matter how big or small it is.
This is because gas molecules move very fast and bump into each other, causing them to spread out evenly.
Structure Of Gas And How Do They Behave
Gas molecules are widely spread apart and move randomly at high speeds due to weak intermolecular forces of attraction. The kinetic theory of gases describes the behavior of gases, which explains how their motion is influenced by temperature, pressure, and volume.
As the temperature of a gas increases, its molecules move faster and collide more frequently, resulting in higher pressure. Gases can also expand to fill their container’s entire volume and can be easily compressed into a smaller volume when subjected to high pressure.
These properties are important in various scientific and industrial processes such as combustion, engine operation, and refrigeration systems.
How Is Gas Measured?
Gas is typically measured in volume units, such as cubic meters or cubic feet, or by weight, such as kilograms or pounds. Gas meters installed in homes and businesses measure the volume of gas consumed.
Gas pipelines and storage tanks may use other methods, such as flowmeters or pressure sensors, to measure gas flow and volume. To determine its value and suitability for different applications, gas quality is also measured, often by its heating value or energy content.
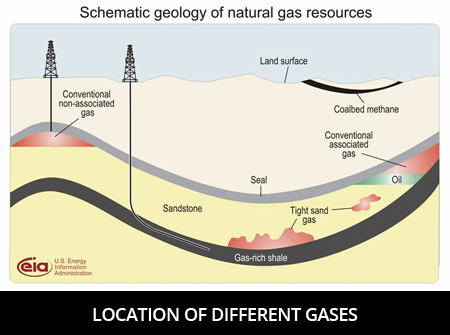
What Are The Different Types Of Gases?
Gases can be categorized based on the number of atoms needed to make them. Pure gases, like neon, are made up of only one atom.
Elemental gases, like hydrogen, are made of two or more of the same atom. Compound gases, like carbon monoxide, are a combination of different types of atoms.
Gases can also be classified based on if they are natural or artificial. Natural gases are an important part of nature and are made by nature. These are common gases like oxygen and nitrogen.
Artificial gases are made by humans from chemical reactions. These gases aren’t helpful to nature and can actually be harmful.
For example, certain artificial gases can damage the ozone layer, which is needed to protect us from the sun.
There are also noble gases, which normally won’t bond with different elements. They can only bond with other elements of the same kind.

Why Are Gases Important?
We already learned that Earth’s atmosphere and the air we breathe are made up of gases, but that’s not the only reason gases are important.
Oxygen and nitrogen can help people who have trouble breathing on their own. Other gases can be used as anesthesia, which keeps people from feeling pain during medical procedures.
Gases can be used for cooking and for generating electricity. They are used in lamps, electric light bulbs, and refrigerators. They help keep our food sterile and safe to eat.
Natural gases are used in the production of paper, glass, and clothing.
Different types of gases are found in balloons (helium), soda (carbon dioxide), gas grills (propane), and lighters (butane). Hydrogen may one day even be used to fuel cars.
These are only some of the many, many ways gases are used every day!
1. Gas Expands
When a gas expands, it basically spreads out and takes up more space than it did before. This happens because the tiny particles that make up the gas, like atoms and molecules, move farther away from each other, and as a result, the gas becomes less dense. When a gas expands, it also causes its pressure to decrease.
This can happen in many ways, such as when a balloon pops, or fuel is burned in an engine. Understanding how gas expansion works is important in the engineering and physics fields. It’s just one of those basic principles we can’t live without!
2. Gas Pressure
Gas pressure is essential in understanding the behavior and importance of gases. The pressure is the force exerted by its molecules on the walls of its container—several factors, including temperature, volume, and the number of gas molecules present, influence it.
Understanding gas pressure is crucial in fields such as chemistry, physics, and engineering, as it helps explain phenomena like the behavior of weather patterns, the operation of combustion engines, and the functioning of the human respiratory system. Accurate measurement and control of gas pressure are vital in many industrial processes, making it an essential parameter to consider.
3. State Changes
Gases are important because they are vital in many natural processes and human activities. For example, oxygen and carbon dioxide are essential for respiration and photosynthesis, respectively, while nitrogen is a crucial fertilizer component.
Gases also undergo phase changes in response to changes in temperature and pressure, transitioning between the three states of matter: gas, liquid, and solid.
When a gas is cooled or compressed, it can condense into a liquid form or freeze into a solid. Conversely, when a liquid is heated or depressurized, it can vaporize into a gas. These phase changes have important implications for everything from weather patterns to industrial processes.

Ozone Layer
The Earth’s atmosphere comprises a complex mixture of gases, including nitrogen, oxygen, argon, carbon dioxide, neon, helium, and methane. These gases are essential to maintaining life on our planet, as they help regulate the temperature, protect us from harmful radiation, and provide the oxygen we need to breathe.
One particular gas that plays a critical role in protecting us from harmful radiation is ozone, which is a form of oxygen that contains three atoms instead of two. The ozone layer is a region of the Earth’s stratosphere where the ozone concentration is highest, and it acts as a shield, absorbing harmful ultraviolet (UV) radiation from the sun.
Unfortunately, human activity has led to the depletion of the ozone layer, primarily through the release of chemicals known as chlorofluorocarbons (CFCs) that react with ozone molecules and break them down. This depletion has led to an increase in skin cancer, cataracts, and other health problems, as well as ecological damage.
To address this problem, the international community came together in 1987 to sign the Montreal Protocol, which banned CFC production and use. Since then, the ozone layer has slowly begun to recover, although it will take many decades to restore it fully.
Are Gases Dangerous?
Gases can be dangerous depending on their properties and concentrations. Some gases, such as carbon monoxide and methane, are flammable and can pose a fire or explosion risk.
Others, such as chlorine and ammonia, are highly toxic and can cause respiratory problems or even death if inhaled in high concentrations. Carbon dioxide, while not harmful, can displace oxygen in enclosed spaces and lead to suffocation.
Handling gases with care and following safety guidelines is important to avoid accidents and health hazards.
Facts About Gas
1. Gas is essential to life on Earth. Oxygen helps produce energy in the human body, and carbon dioxide is used by plants for photosynthesis.
2. Fire is formed from a combination of hot gases.
3. Natural gases are lighter than air.
4. Gases that are usually liquids at room temperature are called “vapor.” “Water vapor” is something you’ve probably heard of before.
5. Some gas particles move so fast that they travel faster than the speed of sound!
6. In 1785, Britain became the first country to use natural gases to light houses and streetlights.
7. Natural gases are odorless, meaning they have no smell, so energy companies add a rotten egg smell so that people will be able to smell gas leaks.
8. Heat causes gases to expand.
9. Natural gases were formed about 100 million years ago.

10. We may not be able to see gases, but they are a huge part of our daily lives. Next time you drink a bubbly soda, blow up a balloon, or even take a deep breath, you’ll know that none of it would be possible without gases.
The Egg Drop Challenge For Kids – How to Do It!
