Isotope Facts
Atoms are the building blocks of life. They make up everything and anything in the world, or even the known universe for that matter. Atoms are made up of protons, neutrons, and electrons.
Every element on the periodic table has its own specific number, which corresponds to the number of protons that the atoms that make up that element will have.
This is also true for the number of electrons that will be found in these atoms since this number always matches the number of protons.
What Are Isotopes?
The only thing that can be different about two atoms from the same element is the number of neutrons that each one contains.
If a set of atoms from the same element has a different number of neutrons, they are referred to as isotopes of that element.
You need to remember, however, that even if a set of atoms has a different number of neutrons, they will still belong to that same element.
But they will be referred to as isotopes of that element, rather than just plain old atoms.
How are Isotopes Named?
As you just read, changing the number of neutrons does not change or even affect the chemistry of the element. This is because neutrons are neutral, or, have no charge.
The only effect that the number of neutrons has on an atom is that they can cause a change in the mass.
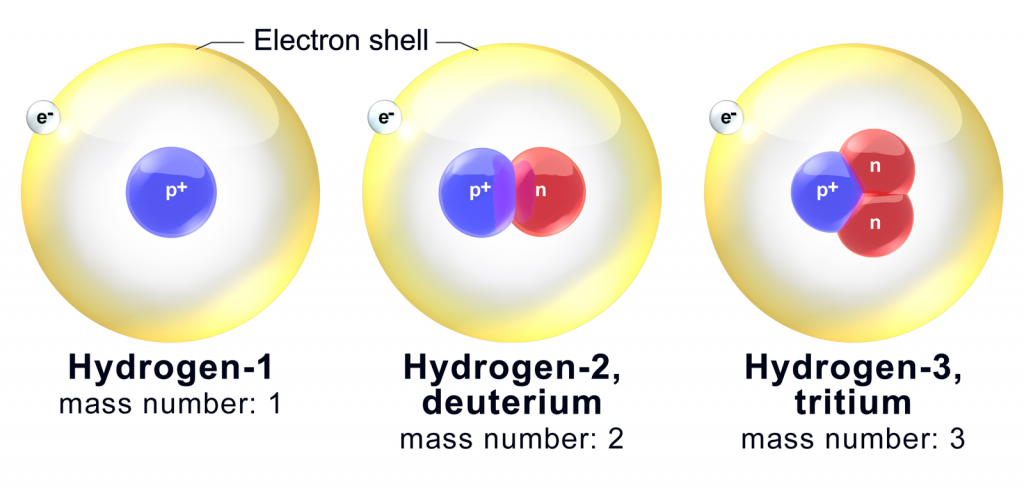
Isotopes, therefore, are identified by their mass, which is the total number of protons and neutrons within the nucleus of the atom.
Isotopes are usually written in one of two ways.
No matter which way an isotope is written, the mass of it is written, which is again the number of protons and neutrons combined.
They can either be written with the mass first:
14C
235U
Or the mass can be written after the element name and a hyphen, like so:
Carbon-14
Uranium-235
Do All Elements Contain Isotopes?
To put it as short as possible, yup! All elements have some isotopes. Hydrogen only has three, which is by far the smallest number out there.
Cesium and Xenon each take the crown for the most naturally occurring isotopes, as they each have 36 that have been observed.
Stable and Unstable Isotopes
When it comes to labeling an isotope as stable or unstable, all you have to do is take a look at how long it hangs around.
If it is only there for a short amount of time before it decays, then it is unstable. Once it has decayed fully, these isotopes will either be a different isotope, or an entirely different element.
These unstable isotopes are considered to be radioactive.
Most of the elements found in nature are made up of stable isotopes.
This type of isotope is resistant to change and will generally stick around for a good long time without decaying.
Tin has the most stable isotope of any naturally occurring substance. It has about ten stable isotopes within its structure.