Copper Facts – What is Copper?
Copper is an element from the periodic table of elements that falls on the first row of the eleventh column in the table.
Copper is known to be a transition metal based on its specific properties.
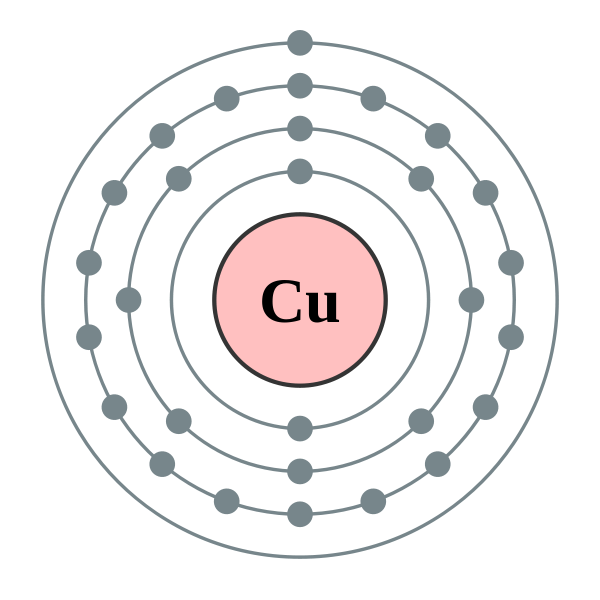
The atomic symbol for copper is Cu, and its atomic number is 29. This means that copper has 29 protons, 29 electrons, and it also has 34 neutrons.
Copper appears in a solid metal form at room temperature, as with most metals, and its melting point is 1084 degrees Celsius.
Its boiling point is extremely high at 2562 degrees Celsius.
With a high 1084 degree melting point, we know that copper is stable in its solid form at very high heat.
Characteristics and Properties
The typical form of copper at room temperature and in its purest form is a solid orange-brass colored metal.
Copper is particularly known for its ability to conduct electricity and heat, making it very commonly used in electrical wiring and manufacturing.
Another bonus property of copper that allows it to be used in industrial settings so often is its ability to be bent and moved and reshaped into new forms.
History
Interestingly, copper was one of the first metal elements to be used as a tool by humans.
However, the discovery of copper happened so long ago in ancient history that today we do not know who the first person to discover copper as an element was.
Copper can be found in the Earth’s outer crust layer, and can often even be found in its purest form, unlike other types of metals that need to be purified before use.
This is the reason that many ancient civilizations were able to use it without having to purify it first.
The reason that copper is typically found in its pure form is that it is very slow to react to oxygen.
Because its reaction is slow and minor, it does not get changed very much from its original form when exposed to air (aside from turning green over time!)
In fact, the primary reaction of copper to air is to turn from a bright orange-ish color to a more dark brown color, and when it is exposed to water, it can corrode into a carbonate that appears green in color.
Interestingly, the United States’ Statue of Liberty is made entirely out of copper, and the reason that the statue appears green today is because of this type of corrosion from the surrounding water (as it is on an island).

Copper is 100% recyclable, and the primary location from which the world gets its copper is from recycling or from mines in Chile, South America.
Like the United States currency, the nickel, which is actually only partially made of nickel, the United States penny is also only partially made of copper anymore.
Before 1982, pennies used to be 95% copper, but after that year, copper increased in price and value, so pennies are now made of 97.5% zinc, with only 2% of their makeup being copper.
Fun Quiz!
- What is the chemical symbol for copper?
- How many protons does copper have?
- What is the form of copper at room temperature?
- What color is copper?
- What happens when copper is exposed to water?
Answers:
- Cu
- 29
- Solid metal, but bendable
- Shiny orange-brown
- It corrodes and turns green over time